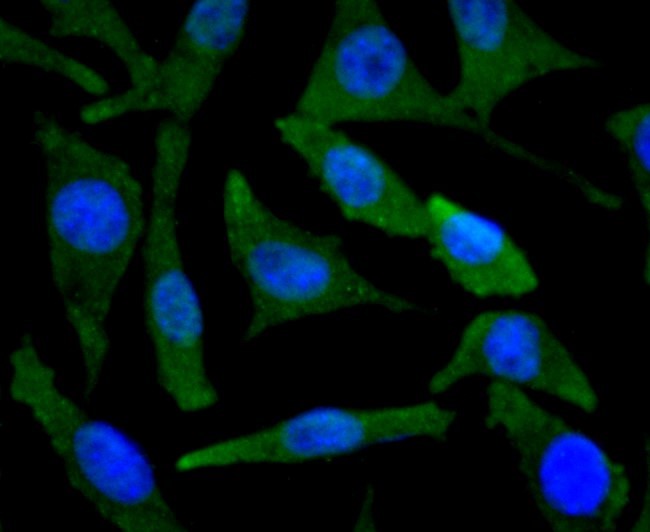
Background:
BNIP3 (Bcl-2/E1B-19kDa interacting protein 3) is a pro-apoptotic mitochondrial protein and Bcl-2 family member that contains a Bcl-2 homology 3 (BH3) domain and a carboxyl-terminal transmembrane (TM) domain. While BNIP3 has a predicted molecular weight of about 22 kDa, it runs anomalously on SDS-PAGE and includes a band of around 60 kDa that may be a dimeric form that is not reduced. BNIP3 associates with anti-apoptotic family members Bcl-2, Bcl-xL, and the adenovirus homologue E1B-19kDa. BNIP3 is distinct from other Bcl-2 family members that contain only the BH3 domain in that the TM domain, and not the BH3 domain, is required for mitochondrial targeting and pro-apoptotic activity. In addition to apoptosis, BNIP3 has been implicated in necrosis and autophagy. In hypoxic conditions, BNIP3 can induce mitochondrial autophagy (mitophagy) by disrupting the Bcl-2-Beclin-1 complex. BNIP3 can also promote mitophagy by triggering the translocation of the E3 ubiquitin ligase Parkin to the mitochondria or by directly binding LC3 on the autophagosome. BNIP3 may also localize to the endoplasmic reticulum (ER) where it can selectively induce the autophagic clearance of ER (ERphagy). Increased expression of BNIP3 under hypoxic conditions is mainly regulated by the transcription factor HIF-1α. Silencing of the BNIP3 promoter by methylation has been observed in several types of cancer cells and may play an important role in their survival.
UniProt ID: Q12983
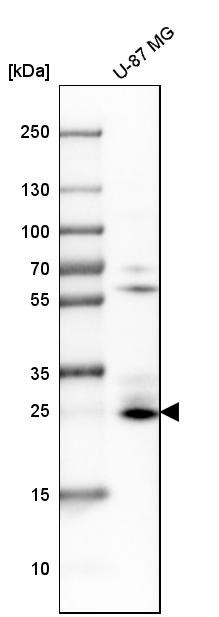
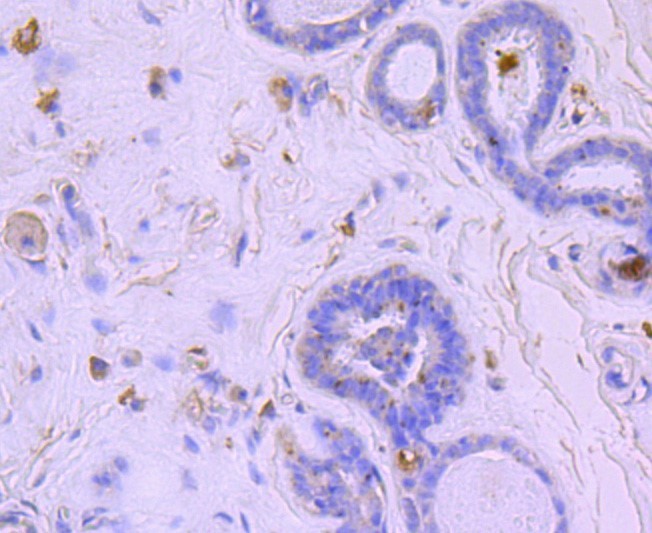 \
\