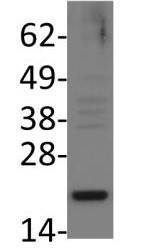
Bad is a proapoptotic member of the Bcl-2 family that promotes cell death by displacing Bax from binding to Bcl-2 and Bcl-xL. Survival factors, such as IL-3, inhibit the apoptotic activity of Bad by activating intracellular signaling pathways that result in the phosphorylation of Bad at Ser112 and Ser136. Phosphorylation at these sites promotes binding of Bad to 14-3-3 proteins to prevent an association between Bad with Bcl-2 and Bcl-xL. Akt phosphorylates Bad at Ser136 to promote cell survival. Bad is phosphorylated at Ser112 both in vivo and in vitro by p90RSK and mitochondria-anchored PKA. Phosphorylation at Ser155 in the BH3 domain by PKA plays a critical role in blocking the dimerization of Bad and Bcl-xL.
UniProt ID: Q92934