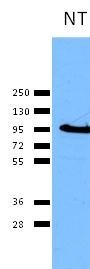
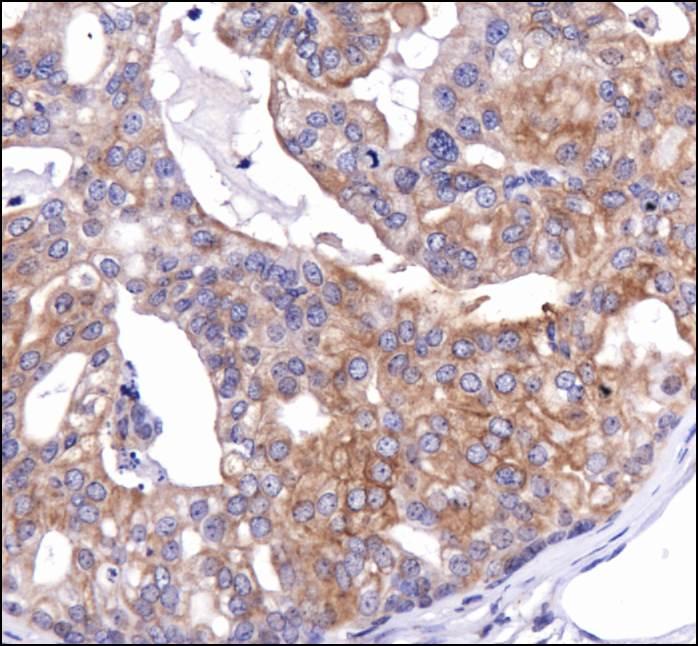
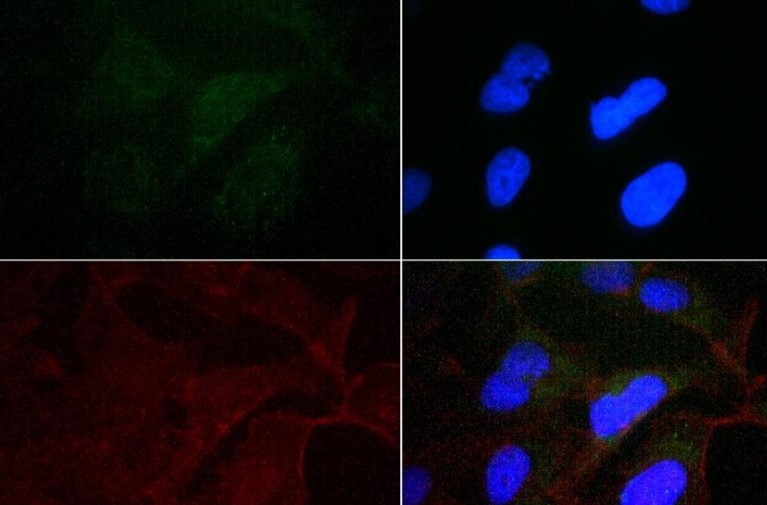
Secretory and transmembrane proteins are synthesized on polysomes and translocate into the endoplasmic reticulum (ER) where they are often modified by the formation of disulfide bonds, amino-linked glycosylation and folding. To help proteins fold properly, the ER contains a pool of molecular chaperones including calnexin. Calnexin was first identified as being involved in the assembly of murine class I histocompatibility molecules. Calnexin is a calcium-binding protein embedded in the ER membrane that retains the newly synthesized glycoproteins inside the ER to ensure proper folding and quality control. The specificity of calnexin for a subset of glycoproteins is defined by a lectin site, which binds an early oligosaccharide intermediate on the folding glycoprotein.
Calnexin is a chaperone protein that assists in protein folding and quality control. Calnexin retains unassembled or unfolded N-linked glycoproteins in the endoplasmic reticulum and ensures that only properly assembled and folded proteins proceed down the secretory pathway. Calnexin antibodies can be used as endoplasmic reticulum markers (ER markers) in immunostaining experiments.