Background:
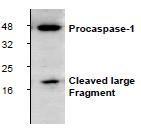
Caspase-1, or interleukin-1ß converting enzyme (ICE/ICEα), is a class I cysteine protease, which also includes caspases -4, -5, -11, and -12. Caspase-1 cleaves inflammatory cytokines such as pro-IL-1ß and interferon-γ inducing factor (IL-18) into their mature forms. Like other caspases, caspase-1 is proteolytically activated from a proenzyme to produce a tetramer of its two active subunits, p20 and p10. Caspase-1 has a large amino-terminal pro-domain that contains a caspase recruitment domain (CARD). Overexpression of caspase-1 can induce apoptosis. Mice deficient in caspase-1, however, have no overt defects in apoptosis but do have defects in the maturation of pro-IL-1β and are resistant to endotoxic shock. At least six caspase-1 isoforms have been identified, including caspase-1 α, β, γ, δ, ε and ζ. Most caspase-1 isoforms (α, β, γ and δ) produce products between 30-48 kDa and induce apoptosis upon over-expression. Caspase-1 ε typically contains only the p10 subunit, does not induce apoptosis and may act as a dominant negative. The widely expressed ζ isoform of caspase-1 induces apoptosis and lacks 39 amino-terminal residues found in the α isoform. Activation of caspase-1 occurs through an oligomerization molecular platform designated the "inflammasome" that includes caspase-5, Pycard/Asc, and NALP1.
UniProt ID: P29466