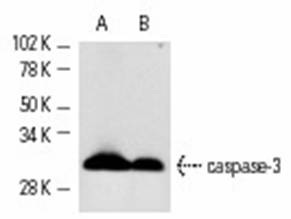
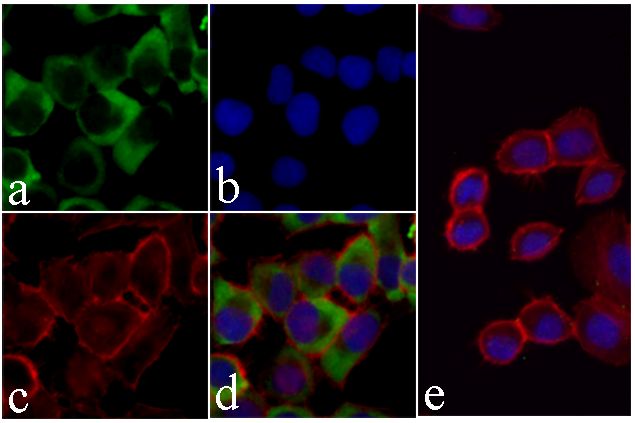
Cleavage by granzyme B, caspase-6, caspase-8 and caspase-10 generates the two active subunits. Additional processing of the propeptides is likely due to the autocatalytic activity of the activated protease. Active heterodimers between the small subunit of caspase-7 protease and the large subunit of caspase-3 also occur and vice versa.
S-nitrosylated on its catalytic site cysteine in unstimulated human cell lines and denitrosylated upon activation of the Fas apoptotic pathway, associated with an increase in intracellular caspase activity. Fas therefore activates caspase-3 not only by inducing the cleavage of the caspase zymogen to its active subunits, but also by stimulating the denitrosylation of its active site thiol.