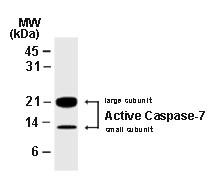
Background:
Apoptosis, or programmed cell death, is a common property of all multicellular organisms. The current dogma of apoptosis suggests that the components of the core cell-death machinery are integral to cells and widely conserved across species. Caspases, a family of cysteinyl aspartate-specific proteases, are integral components of the cell death machinery (reviewed in Siegal, 2006; and Lavrik et al, 2005). They play a central role in the initiation and execution of apoptotic cell death and in inflammation. Caspases are typically divided into 3 major groups, depending on the structure of their prodomain and their function. Group 1: inflammatory caspases (caspases 1, 4, 5, 11, 12, 14). Group II: initiator of apoptosis caspases (caspases 2, 8, 9). Group II: effector caspases (caspases 3, 6, 7). Caspases are synthesized as zymogens (inactive pro enzyme precursors which require a biochemical change to become active enzymes) with an N-terminal prodomain of variable length followed by a large subunit (p20) and a small subunit (p10). Caspases are activated through proteolytic cleavage at specific asparagine residues that are located within the prodomain, the p10, and p20 subunits. Activation results in the generation of mature active caspases that consist of the heterotetramer p202-p102. Active caspases mediate cell death and inflammation through cleavage of particular cellular substrates that are involved in these processes. The Active/Cleaved Caspase-7 polyclonal antisera recognizes the large (approx. 17-22 kDa) and small (approx. 10 kDa) subunits of active/cleaved caspase-7. Whereas the antisera has a strong preference for active/cleaved caspase-7, in some cell or tissue systems or techniques the antisera may also recognize the proform of Caspase-7 (approx. 35 kDa).
UniProt ID: P55210