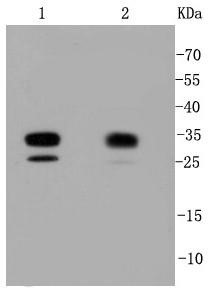
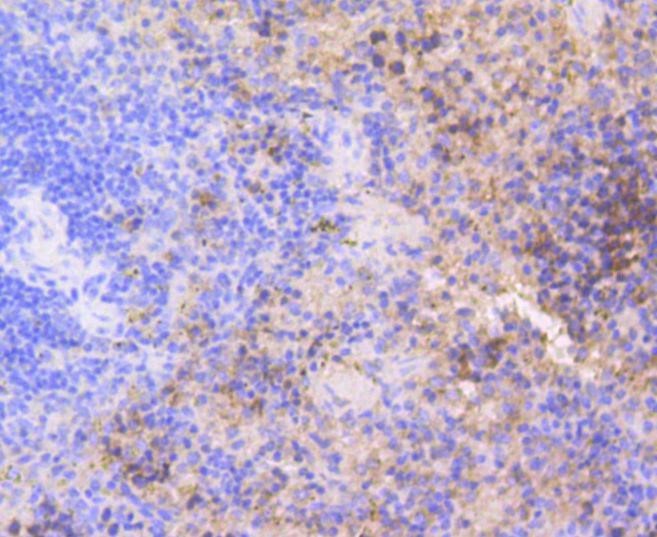
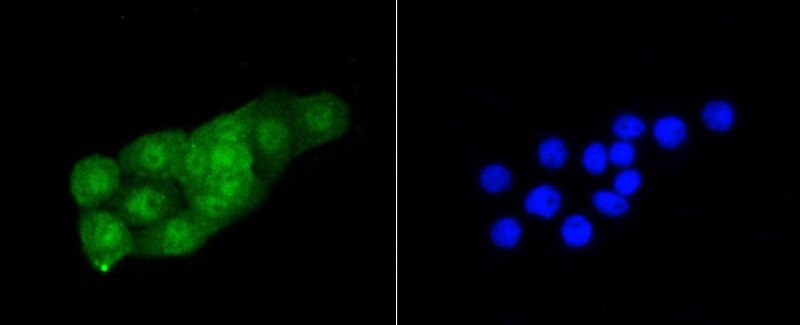
Background:
Cyclin-dependent kinase 2 (p33CDK2) is an important component of the cell cycle machinery. Like p34cdc2, kinase activity is regulated by phosphorylation state as well as association with a cyclin subunit and a CDK inhibitor. Inhibitory phosphorylation occurs on Thr14 and Tyr15. Inhibition of CDK2-cyclin complexes can also be attributed to association with p27 Kip1 and p21 Waf1/Cip1. Activation of CDK2 complexes requires dephosphorylation of Thr14 and Tyr15 by cdc25 phosphatase and phosphorylation of Thr160, which is mediated by CAK, a complex of CDK7 and cyclin H. CDK2/cyclin E kinase activity is important for the G1 to S transition and phosphorylation of the Rb protein. During S-phase, active CDK2/cyclin A complexes predominate and phosphorylate E2F and the active CDK2 complex persists in the nucleus throughout G2.
UniProt ID: P24941