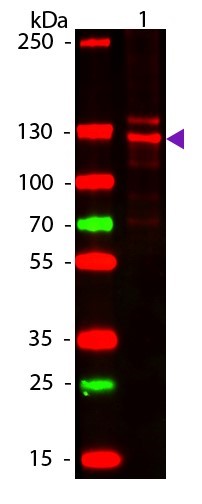
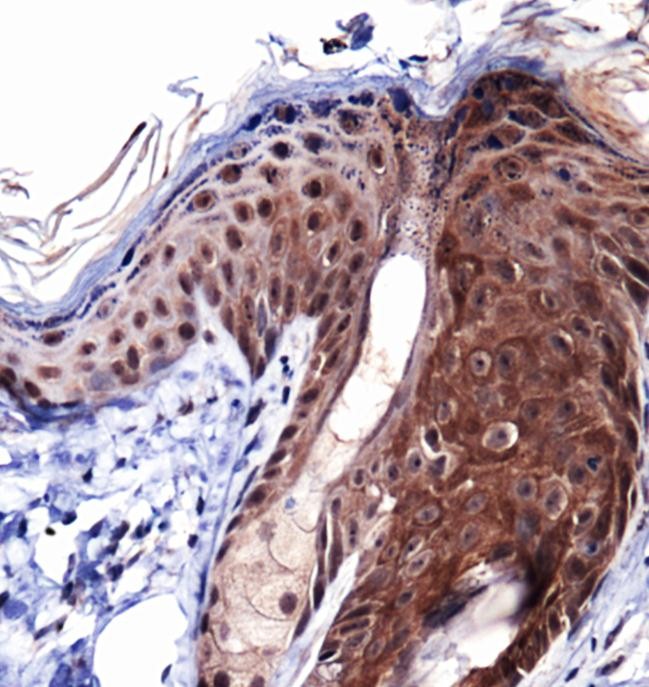
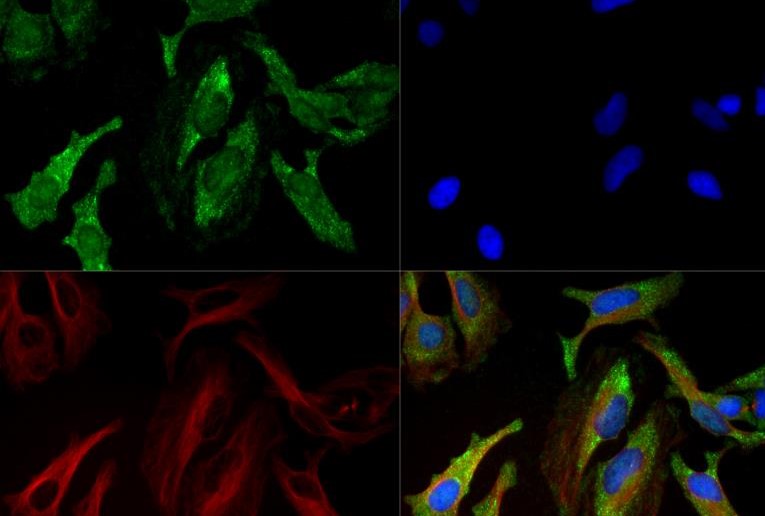
Type 1 collagen is the most abundant collagen in many human tissues, including bone, skin, and tendons. It is a trimeric complex comprised of two molecules of COL1A1 (alpha-1 type 1 collagen) and one molecule of COL1A2 (alpha-2 type 1 collagen). The expression levels of COL1A1 are regulated by multiple mechanisms, including mRNA stability, translation, and posttranslational modification. Overexpression of COL1A1 has been positively associated with tissue fibrosis disorders, including systemic sclerosis, while loss-of-function mutations in the COL1A1 gene are a major causative factor for osteogenesis imperfecta (brittle bone disease). Notably, COL1A1 expression levels have also been associated with tumor development in gastric, lung, thyroid, and breast cancers. Research studies suggest that upregulation of COL1A1 can generate a modified extracellular matrix environment that promotes cancer cell survival, proliferation, metastasis, and invasion.
Collagen-1 (also known as collagen alpha, COL1A1, and alpha-1 type I collagen) is the largest component of fibrillar collagen found in cartilage and connective tissues, and synthesizes by fibroblasts, osteoblasts, and odontoblasts. Trimers are formed by combing two alpha one chains and one alpha 2 chain. Mutations of the gene are found in Ehlers-Danlos syndrome, as well as osteogenesis imperfecta. Over 50 mutations have been found in these diseases, including deletions, insertions, splice variants, and substitutions.
UniProt ID:
P02452