Background:
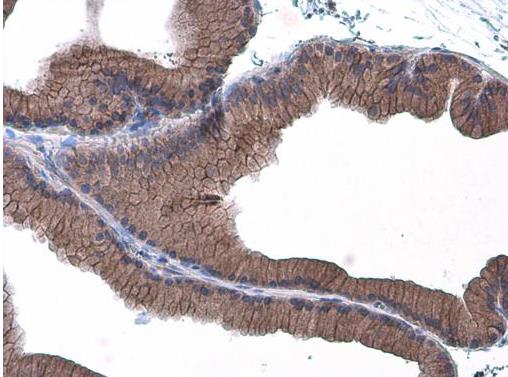
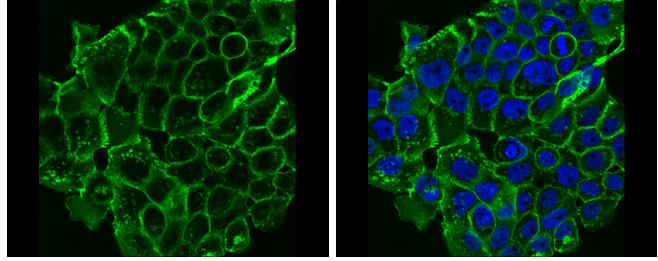
Cadherins are a superfamily of transmembrane glycoproteins that contain cadherin repeats of approximately 100 residues in their extracellular domain. Cadherins mediate calcium-dependent cell-cell adhesion and play critical roles in normal tissue development. The classic cadherin subfamily includes N-, P-, R-, B-, and E-cadherins, as well as about ten other members that are found in adherens junctions, a cellular structure near the apical surface of polarized epithelial cells. The cytoplasmic domain of classical cadherins interacts with β-catenin, γ-catenin (also called plakoglobin), and p120 catenin. β-catenin and γ-catenin associate with α-catenin, which links the cadherin-catenin complex to the actin cytoskeleton. While β- and γ-catenin play structural roles in the junctional complex, p120 regulates cadherin adhesive activity and trafficking. Investigators consider E-cadherin an active suppressor of invasion and growth of many epithelial cancers. Research studies indicate that cancer cells have upregulated N-cadherin in addition to loss of E-cadherin. This change in cadherin expression is called the "cadherin switch." N-cadherin cooperates with the FGF receptor, leading to overexpression of MMP-9 and cellular invasion. Research studies have shown that in endothelial cells, VE-cadherin signaling, expression, and localization correlate with vascular permeability and tumor angiogenesis. Investigators have also demonstrated that expression of P-cadherin, which is normally present in epithelial cells, is also altered in ovarian and other human cancers.
UniProt ID:
P12830