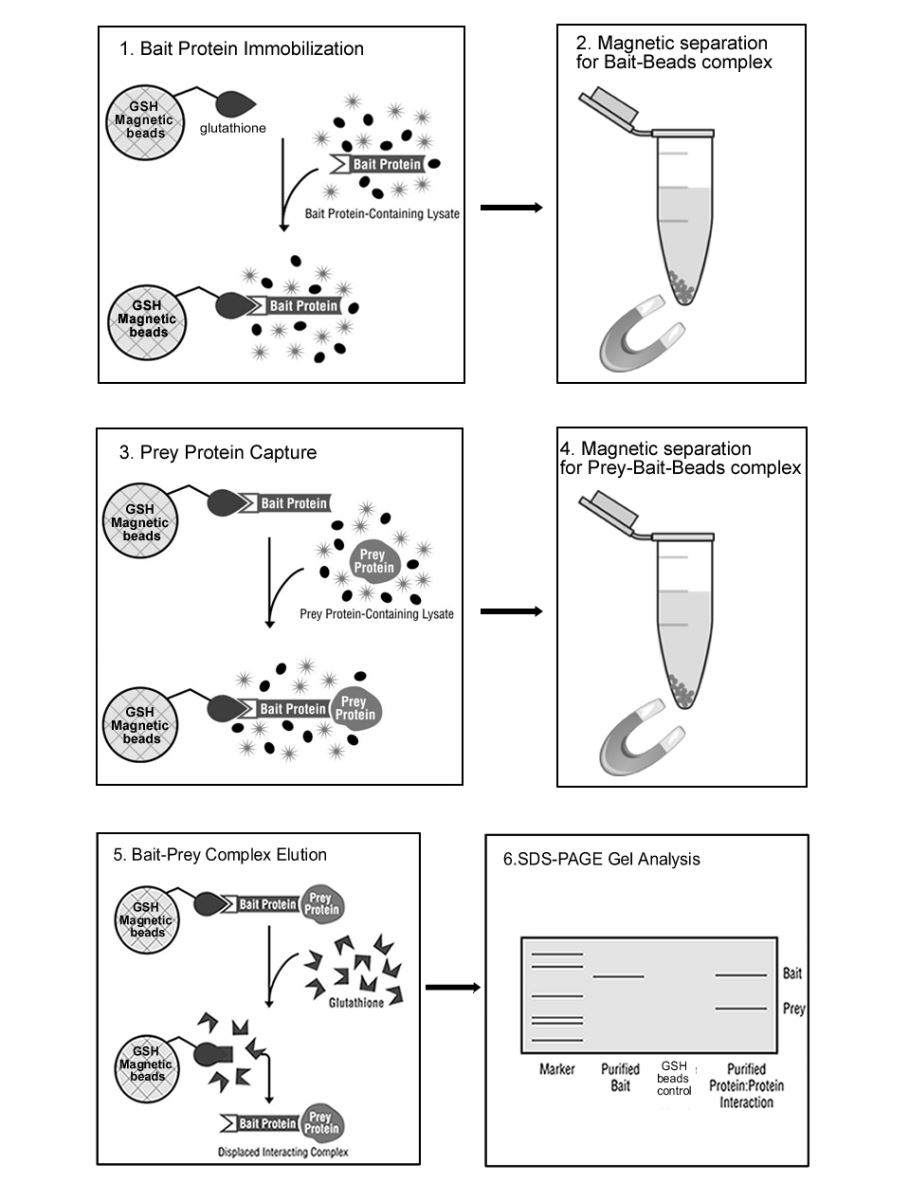
The glutathione S-transferase (GST) pull-down technique has become an invaluable tool for the life scientist interested in protein chemistry.
The basic pull-down assay is an in vitro technique that consists of a GST-tagged bait protein (the
researcher’s protein of interest) that can be used to identify putative binding partner(s) (the prey). The bait protein, purified from an appropriate expression system (e.g., Escherichia coli or baculovirus-infected insect cells), is immobilized to glutathione beads. The bait serves as the secondary affinity support for confirming a previously suspected protein partner or for identifying new protein partners to the bait. Prey protein can be obtained from multiple sources, including previously purified proteins, cell lysates or in vitro transcription/translation reactions.
Protein:protein interactions can be visualized by SDS-PAGE and associated detection methods depending on the sensitivity requirements of the interacting proteins. These methods include coomassie, silver, zinc staining and Western blotting.