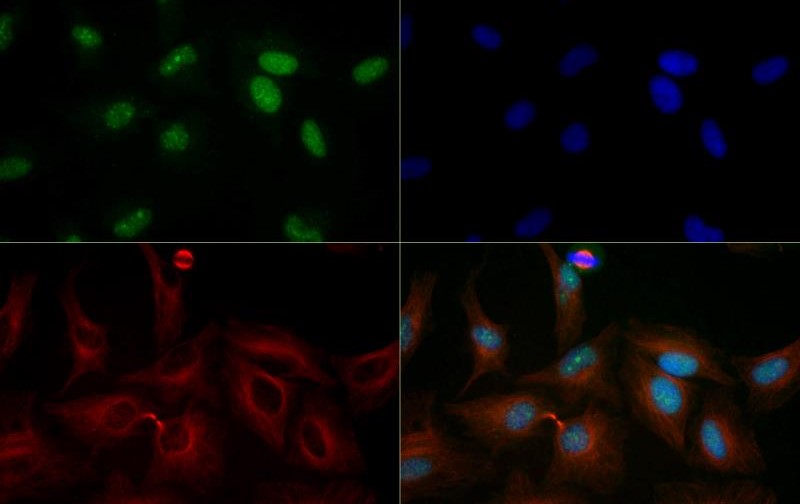
Acetylation of the histone tail causes chromatin to adopt an "open" conformation, allowing increased accessibility of transcription factors to DNA. The identification of histone acetyltransferases (HATs) and their large multiprotein complexes has yielded important insights into how these enzymes regulate transcription. HAT complexes interact with sequence-specific activator proteins to target specific genes. In addition to histones, HATs can acetylate nonhistone proteins, suggesting multiple roles for these enzymes. In contrast, histone deacetylation promotes a "closed" chromatin conformation and typically leads to repression of gene activity. Mammalian histone deacetylases can be divided into three classes on the basis of their similarity to various yeast deacetylases. Class I proteins (HDACs 1, 2, 3, and 8) are related to the yeast Rpd3-like proteins, those in class II (HDACs 4, 5, 6, 7, 9, and 10) are related to yeast Hda1-like proteins, and class III proteins are related to the yeast protein Sir2. Inhibitors of HDAC activity are now being explored as potential therapeutic cancer agents.
Histone deacetylase 4 (HDAC4), also known as HD4, KIAA0288, AHO3, BDMR, HDACA, and HA6116, is crucial for transcription regulation, cell cycle progression and developmental events. HDAC4 also plays a critical role in the deacetylation of lysine residues in the core histones, which results in the production of a tag for epigenetic repression. HDAC4 is a member of the class II histone deacetylase/AcuC/AphA family and has been linked to nucleosomal condensation. The HDAC4 protein is expressed ubiquitously throughout the body and can be found in both the nucleus and cytoplasm. HDAC4 gathers in the nuclei of myotubes during muscle cell differentiation, which indicates HDAC4 plays a role in muscle differentiation. HDAC4 is exported to the cytoplasm based on the interactions with a 14-3-3 chaperone protein and its phosphorylation at Ser-246, Ser-467 and Ser-632 by CaMK4 and SIK1. Mutations in HDAC4 can cause brachydactyly-mental retardation syndrome (BDMR), which resembles Albright hereditary osteodystrophy. Recent research has found that HDAC4 regulates HIF-1, which influences cancer cell production and reaction to hypoxia making HDAC4 a possible protein of interest in cancer treatment (PMID: 21917920). Further research has found that HDAC4 affects the growth of cancerous cells in the prostate (PMID: 19013255). Other areas of research, involving knock-out studies, have found a link between HDAC4 and long term memory formation.
UniProt ID:
P56524