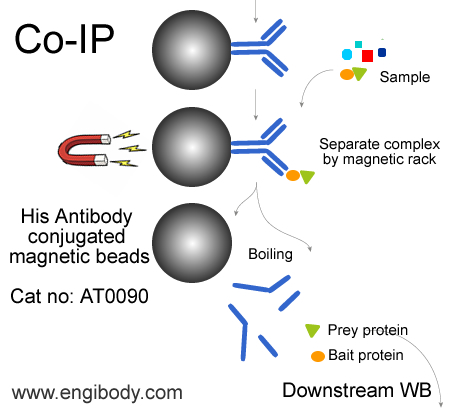
Protocol for Immunoprecipitation (IP/CoIP) of 6xHis-Fusion Proteins from Mammalian Cell Lysate
This protocol is intended for immunoprecipitation of 6xHis- tagged fusion protein for analysis by western immunoblot or activity assay.
Solutions and Reagents
1X Cell Lysis Buffer: 50 mM Tris (pH 7.5), 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1% Triton X-100. Recommend adding 1 mM PMSF before use.
1X Wash Buffer: TBS (50 mM Tris HCl, 150 mM NaCl, pH 7.5)
5X SDS Sample Loading Buffer
1. Preparing Cell Lysates
1. To harvest cells under nondenaturing conditions, remove media and rinse cells once with ice-cold PBS. Remove PBS and add 0.5 mL-1mL 1X ice-cold cell lysis buffer (added Protease Inhibitor Cocktail and PMSF) to each plate (10 cm,106-107 cells) and incubate the plates on ice for 30-40 minutes with rotation at 4°C.
2. Scrape Lysed cells off the plates and transfer to microcentrifuge tubes. Keep on ice.
3. Sonicate samples on ice three times for 5 seconds each (optional step).
4. Microcentrifuge for 10 minutes at 4°C, 14,000 x g, and transfer the supernatant to a new tube. If necessary, lysate can be stored at –80°C.
Attention: If the target protein is nuclear protein, please use RIPA lysis buffer to lyse the cell, and add PIC, 1mM PMSF, 2.5mM Mgcl2 and 1mg/ml DNase I.
2. Equilibrate Beads
1. Resuspend the beads by inverting the product tube or gently pipetting up and down. Do not Vortex the beads.
2. Pipette 25 µL bead slurry into an EP tube (1.5 mL),then add 1mL ice-cold wash buffer. Shake gently by your hand for 1-2 minutes. Do not vortex the beads.
3. Magnetic separation for 60 seconds.
4. Discard supernatant and repeat wash three times.
3. Immunoprecipitation
1. Take 500 μL cell lysate and add 25 µL of the antibody conjugated magnetic beads suspension, incubate with rotation for 1-3 hours at 4°C or overnight at 4°C.
4. Wash the protein-antibody-beads complex
NOTE: if Co-IP interacting proteins are researched, please reduce the number of washes, and lower the ionic strength of the wash buffer.
Add 1.0 mL of Wash Buffer and suspend the beads complex, magnetic separation, then discard the supernatant. Repeat above steps at least four times.
5. Elution for Downstream Analysis
Elution of the 6xHis- fusion proteins - Two elution methods are recommended according to protein characteristics or further usage:
Option A: Native Elution
Elution under acidic conditions with 0.2 M glycine (pH 2.5). This is a fast and efficient elution method. Neutralization of the eluted proteins with neutralizing buffer (1 M Tris, pH 10.4) may help preserve its activity. Neutralizing buffer needs to be placed in the collection tube in advance.
Attention: It is necessary to make a preliminary experiment in advance to determine how much neutralization buffer is needed to neutralize glycine (PH 2.5)
Option B: Denaturing Elution for SDS-PAGE
Elution with sample loading buffer under denaturing conditions for gel electrophoresis and immunoblotting.
A: Elution with 0.2 M Glycine (pH 2.5) - The procedure should be performed at room temperature.
Note:Do not leave the beads in this buffer more than 20 minutes.
1. Add 50 µL of 0.2 M Glycine (pH 2.5), to each sample and control beads complexes.
2. Incubate the samples and controls with constantly pipette up and down for 1-3 minutes at room temperature. (Note: Do not turn the tube upside down to prevent the complex from sticking to the tube wall)
3. Place tube in the appropriate magnetic separator to collect the beads. Transfer the supernatants to fresh tubes containing about 5 µL of neutralizing buffer. Be careful not to transfer any beads.
4. Repeat steps 1 – 3 in order to improve elution efficiency, pooling eluates in same tube or collect eluate through different tubes.
6. For immediate use, store the eluates at 2-8 °C. Store at –20 °C for long term storage.
B: Elution with SDS-PAGE Sample Loading Buffer
1. Resuspend each sample with 30 µL 1X SDS sample loading buffer (6 µL 5X SDS sample loading buffer can be added into 24 µL cell lysis buffer). Vortex.
2. Boil the sample and control tubes for 10 minutes at 95 – 100°C.
3. Place tubes in the magnetic separator to collect the beads. Transfer the supernatants to fresh tubes. The samples and controls are ready for loading on SDS-PAGE and immunoblotting using anti- 6xHis or specific antibodies against the fusion protein.