Background:
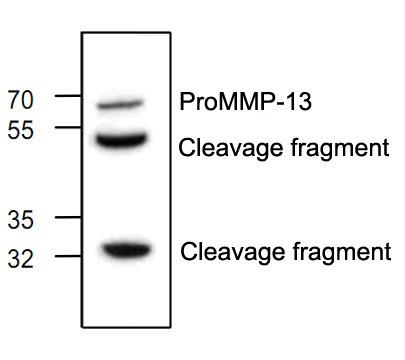
MMP13 (collagenase 3) belongs to the matrix metalloproteinase (MMP) superfamily of enzymes that targets many extracellular proteins, including other proteases, growth factors, cell surface receptors, and adhesion molecules. MMP13 is a member of a subgroup of collagenases (including MMP1, MMP8, and MMP18) that play an even more important function targeting fibrillar collagen. MMP13 is synthesized as a latent proenzyme, and proteolytic removal of the inhibitory propeptide domain is required for enzyme activation. MMP13 protein levels are regulated at the transcriptional level, via specific transcription factors and via promoter DNA methylation. MMP13 preferentially cleaves Type II collagen, and research studies have shown that aberrant upregulation of MMP13 activity can lead to cartilage loss and osteoarthritis. In addition, MMP13 has been shown to promote cancer development, in part through enhancing tumor angiogenesis and metastases, suggesting that collagenase activity may serve as a useful marker of tumor progression.
UniProt ID: P45452