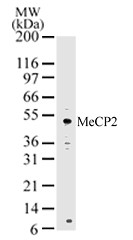
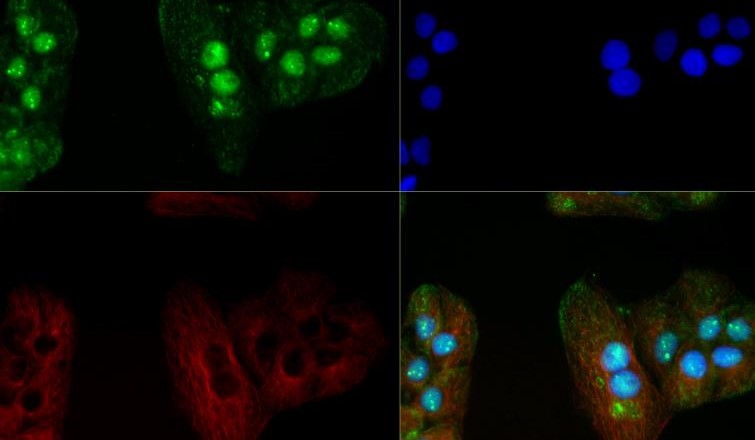
Methyl-CpG-binding protein 2 (MeCP2) is the founding member of a family of methyl-CpG-binding domain (MBD) proteins that also includes MBD1, MBD2, MBD3, MBD4, MBD5 and MBD6. Apart from MBD3, these proteins bind methylated cytosine residues in the context of the di-nucleotide 5´-CG-3´ to establish and maintain regions of transcriptionally inactive chromatin by recruiting a variety of co-repressor proteins. MeCP2 recruits histone deacetylases HDAC1 and HDAC2, and the DNA methyltransferase DNMT1. MBD1 couples transcriptional silencing to DNA replication and interacts with the histone methyltransferases ESET and SUV39H1. MBD2 and MBD3 co-purify as part of the NuRD (nucleosome remodeling and histone de-acetylation) co-repressor complex, which contains the chromatin remodeling ATPase Mi-2, HDAC1 and HDAC2. MBD5 and MBD6 have recently been identified and little is known regarding their protein interactions. MBD proteins are associated with cancer and other diseases; MBD4 is best characterized for its role in DNA repair and MBD2 has been linked to intestinal cancer. Mutations in the MeCP2 gene cause the neurologic developmental disorder Rett Syndrome. MeCP2 protein levels are high in neurons, where it plays a critical role in multiple synaptic processes. In response to various physiological stimuli, MeCP2 is phosphorylated on Ser421 and regulates the expression of genes controlling dendritic patterning and spine morphogenesis. Disruption of this process in individuals with altered MeCP2 may cause the pathological changes seen in Rett Syndrome.
UniProt ID:
P51608