Cleavage buffer: 50mM Tris, pH8.0, 150mM NaCl, 2.5mM CaCl2, 0.1% β-ME
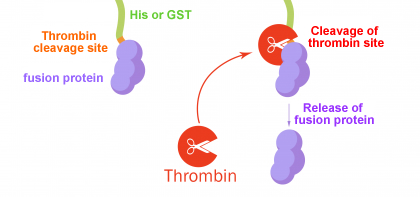
Thrombin Cleavage of Free Eluted Fusion protein
First you have to calibrate amount of thrombin, temperature and length of incubation, taking in mind that one unit cleaves theoretical 100ug protein in 16hr at 22°C in buffer.
You can incubate enough eluate to see on PAGE-SDS gels (~3ug) with 0.01, 0.03 & 0.06 Units of thrombin 2hr, 4hr, 6hr & ON at 22°C (or RT). Stop the reaction with PAGE-SDS sample buffer + 1mM PMSF and keep immediately at -20°C until use. Analyze PAGE-SDS gels versus a control of non-cleavaged protein.
Longer incubation, more enzyme or higher temperature will increase cleavage; while lower incubation, less enzyme or lower temperature will decrease cleavage.
As a general protocol you can use:
1. To the eluate from either batch or column purification, add 10ul of thrombin solution (10 cleavage units) per mg fusion protein.
2. Mix gently and incubate at RT for 2-16hrs
3. Once digestion is complete you can stop protease cleavage with 1mM PMSF or AEBSF (more stable) and check results by PAGE-SDS gels or immediately separate the cleavage products by chromatography
4. When a satisfactory condition is found, scale-up the reaction proportionally.
Avoid the presence of serine protease inhibitors (like PMSF or AEBSF) during cleavage
Thrombin Cleavage of Fusion protein Bound to column matrix
First you have to calibrate the amount of thrombin, temperature and length of incubation, taking in mind that one unit cleaves theoretical 100ug protein in 16hr at 22°C in buffer or one unit cleaves theoretical 20ul of bed volume saturated resin in 16hr at 22°C in buffer.
You can incubate 20ul of bed volume saturated resin with 0.1, 0.5, 1 & 2 Units of thrombin (in 20ul total PBS) 2hr, 4hr, 6hr & ON at 22°C (or RT). Stop reaction by spinning for 4min 3000rpm, separate supernatant from beads, and add PAGE-SDS sample buffer + 1mM PMSF to the supernatant, and keep immediately at -20°C until use. Analyze PAGE-SDS gels versus a control of non-cleavaged protein (eluted non cleaved protein).
Longer incubation, more enzyme or higher temperature will increase cleavage; while lower incubation, less enzyme or lower temperature will decrease cleavage.
As a general protocol you can use:
1. For 1ml of bed volume saturated resin, mix 50Units of thrombin solution in 1ml PBS. Gently swirl to mix. Shake or rotate at 22°C (or RT) 2-16hrs
2. Spin the suspension 4min 3000rpm to pellet the beads. Keep supernatant aside. You can stop protease cleavage with 1mM PMSF or AEBSF (more stable).
3. Extract beads twice or more with 1ml PBS or buffer. Keep each supernatant aside.
4. As a control you can elute the remaining uncleaved protein (still attached to the resin through the GST) by extraction several times with elution buffer.
5. Check results by PAGE-SDS gels or immediately separate the cleavage products by chromatography.
6. When a satisfactory condition is found, scale-up the reaction proportionally.
Thrombin Removal with pAmino benzamidine-Agarose
50 units of Thrombin can be removed by shaking or rotating at 22°C (or RT) 30 min with 100ul with pAminoBenzamidine-Agarose (SIGMA #A 7155).