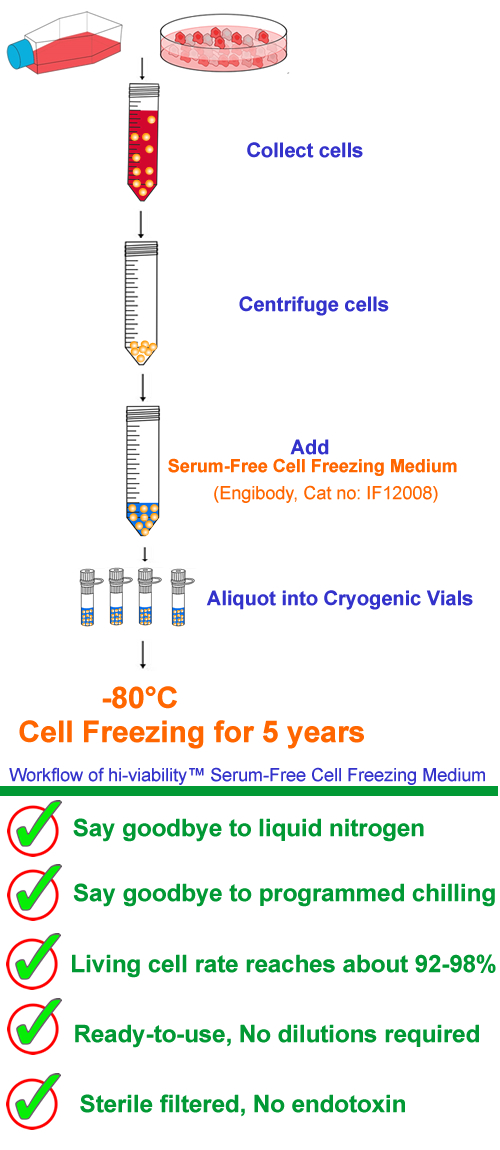
A. Cell cryopreservation steps
1. Collect log-phase adherent cells or suspension cells in tubes by general methods.
2. Determine the required number of cryopreserved cells according to the density of the cultured cells and the size of the cryovial.
3. Put the required number of cell suspensions into a centrifuge tube, centrifuge at 1000 rpm for 5 minutes to collect the cultured cell pellet, and completely discard the supernatant in the centrifuge tube.
4. Add an appropriate amount of Hi-viability serum-free cell freezing medium to the centrifuge tube to make the cell concentration about 5×105-1×107/ml. Gently mix cells to make a cell mixture.
5. Dispense the cell mixture in the centrifuge tube into labeled cryopreservation tubes, 1ml or 1.5ml per tube is recommended.
6. Put the subpackaged cell cryopreservation tube directly into the -80 ultra-low temperature freezer, which can be frozen for a long time (more than about 5 years).
7(optional step). If you want to store in liquid nitrogen for a long time, you need to put it in a -80°C refrigerator for at least one day before moving it to a liquid nitrogen tank for storage.
B. Cryopreserved cell recovery steps
1. Get the frozen cells from the refrigerator and immediately place them in a 37°C water bath to quickly thaw them.
2. After the cell mixture in the cryovial is completely thawed, immediately add 1ml of cell culture medium into tube to mix with the cells, transfer the mixture to a centrifuge tube containing about 5ml of the cell culture medium, 1000rpm, 5 minutes. Collect the frozen cell pellet by centrifugation and discard the supernatant (be careful not to discard the cell pellet).
3. Add an appropriate amount of fresh cell culture medium, slowly add it to the cell pellet with a pipette, mix gently, and transfer the cell mixture to the prepared culture vessel.
4. After microscopic examination of the cells, the cells can be generally cultured according to the needs of their respective researches.