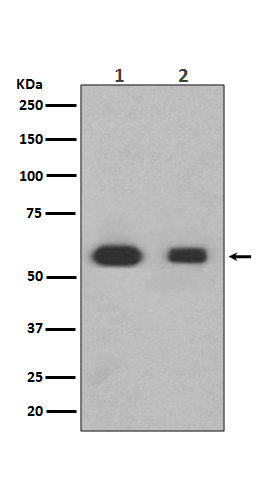
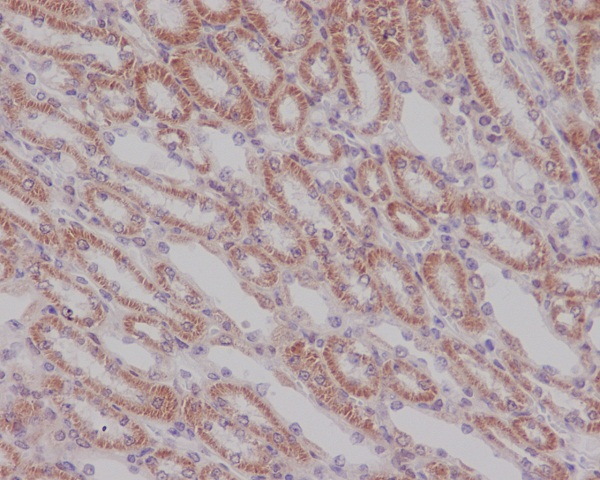
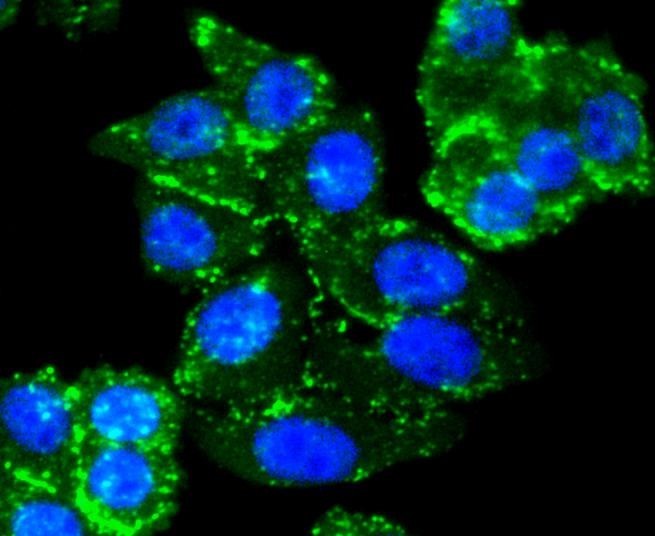
Members of the Smad family of signal transduction molecules are components of a critical intracellular pathway that transmit TGF-β signals from the cell surface into the nucleus. Three distinct classes of Smads have been defined: the receptor-regulated Smads (R-Smads), which include Smad1, 2, 3, 5, and 8; the common-mediator Smad (co-Smad), Smad4; and the antagonistic or inhibitory Smads (I-Smads), Smad6 and 7. Activated type I receptors associate with specific R-Smads and phosphorylate them on a conserved carboxy terminal SSXS motif. The phosphorylated R-Smad dissociates from the receptor and forms a heteromeric complex with the co-Smad (Smad4), allowing translocation of the complex to the nucleus. Once in the nucleus, Smads can target a variety of DNA binding proteins to regulate transcriptional responses.
Following stimulation by TGF-β, Smad2 and Smad3 become phosphorylated at their carboxyl termini (Ser465 and 467 on Smad2; Ser423 and 425 on Smad3) by TGF-β Receptor I. Phosphorylated Smad 2/3 can complex with Smad4, translocate to the nucleus and regulate gene expression.