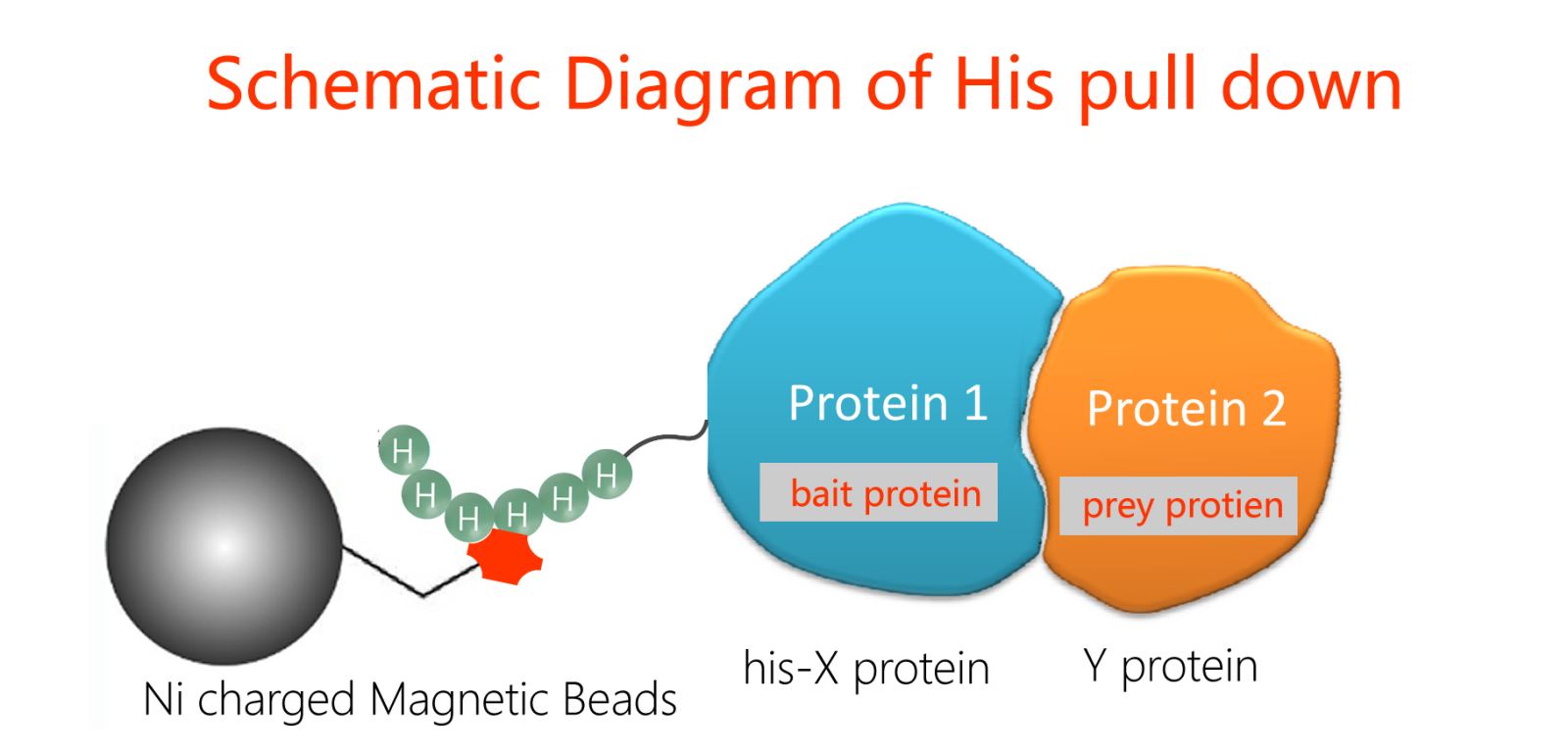
Protein-Protein Interactions (PPIs)
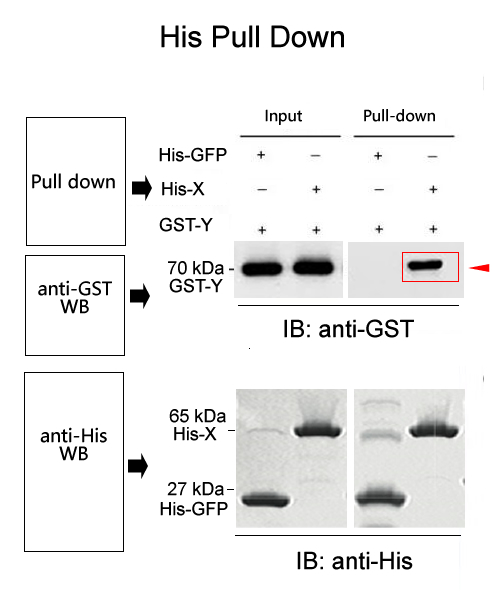
His pull down is the fusion of a known protein with his epitope, which binds affinity to Ni magnetic beads through a his tag. Simply put, we assume that there may be interaction between X protein and Y protein. Purified fusion his-X protein, purified Y protein, and Ni magnetic beads that can specifically bind to his tag are mixed together and incubated for a certain period of time. Then, unbound proteins are washed away, and bait protein X and prey protein Y are washed off, followed by SDS-PAGE electrophoresis. Through Western Blot detection results, the corresponding bands of his-X protein and Y protein can be seen, indicating that X protein and Y protein are pulled down by his-X due to their interaction.
Note: Western Blot, MS mass spectrometry and other experiments can be connected downstream. Western Blot was used to validate known interacting proteins, while MS mass spectrometry was used to screen for the discovery of unknown interacting proteins.