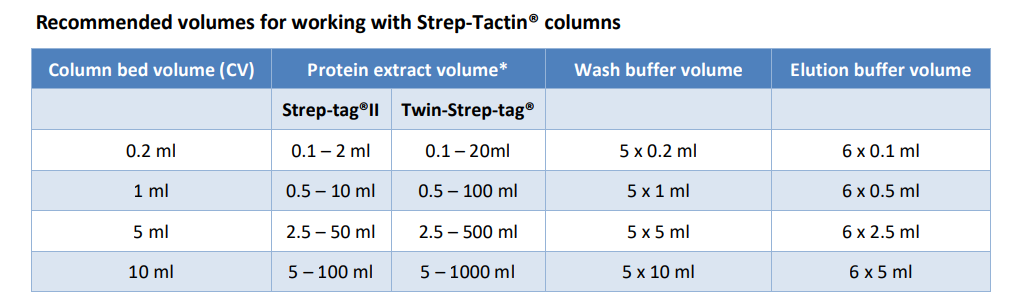
The Strep-tag® purification system is based on the highly selective binding of engineered streptavidin, called Strep-Tactin®, to Strep-tag®II fusion proteins. This technology allows one-step purification of almost any recombinant protein under physiological conditions, thus preserving its biological activity. The Streptag® system can be used to purify functional Strep-tag®II proteins from any expression system including baculovirus, mammalian cells, yeast, and bacteria. Unique Strep-Tactin® affinity columns have been developed for this purpose and the corresponding operating protocol is described below. Streptag®:Strep-Tactin® affinity purification should not be performed discontinuously in batch mode which would result in significantly reduced protein purity and yield in comparison to column chromatography.
Further, prolonged batch incubations increase the risk of proteolytic degradation of the target protein including cleavage of the tag. Because of its small size, Strep-tag® generally does not interfere with the biological activity of the fusion partner. Thus, removal of the tag becomes superfluous.
The Twin-Strep-tag® is a dimeric version of the Strep-tag®II and therefore binds with the same selectivity to Strep-Tactin® but with a higher affinity. This increased affinity allows the purification of Twin-Streptagged proteins even from batch or cell culture supernatants with excellent yields. In addition the TwinStrep-tag® tolerates higher amounts of detergents and salts in buffers compared to Strep-tag®II. Since the overall conditions for Strep-tag®II and Twin-Strep-tag® are the same, the following protocol can be used for both tags.