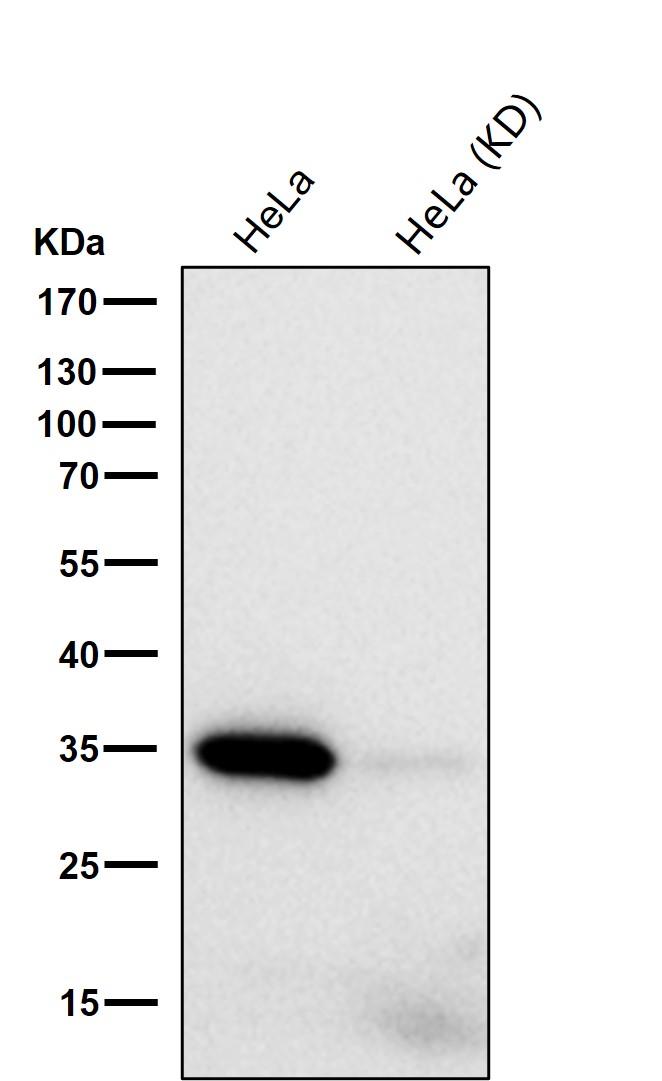
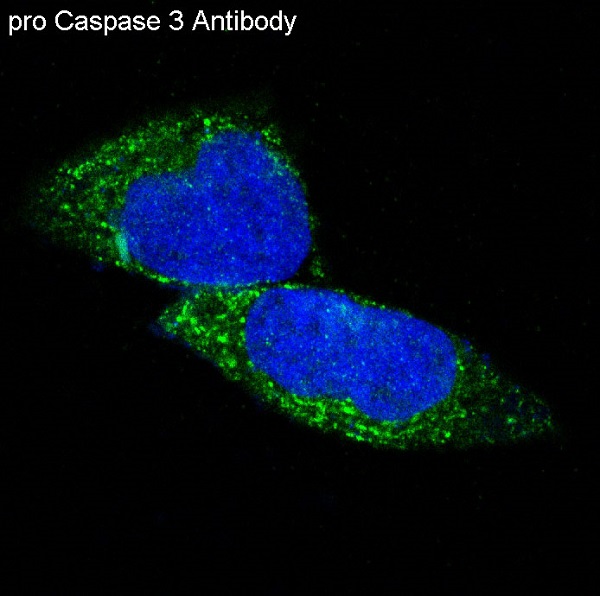
Background:
The pro caspase-3 is precursor (32kDa), it is first cleaved at Asp175-Ser176 to produce the p12 subunit
and the p19 protein. Subsequently, the p19 protein is cleaved at Asp28-Ser29 to generate the
mature p17 subunit. The active caspase-3 enzyme is a heterodimer composed of two p17 and
two p12 subunits.
At the onset of apoptosis, caspase-3 proteolytically cleaves PARP at an Asp216-Gly217 bond.
During the execution of the apoptotic cascade, activated caspase-3 releases SREBP from the
membrane of the ER in a proteolytic reaction that is distinct from their normal steroldependent
activation. Caspase-3 cleaves and activates SREBPs between the basic helixloop-helix leucine
zipper domain and the membrane attachment domain. Caspase-3 also cleaves and activates
caspase-6, -7 and -9. The human caspase-3 gene encodes a cytoplasmic protein that is highly
expressed in lung, spleen, heart, liver, kidney and cells of the immune system.
UniProt ID: P42574