Enterokinase is a specific protease that cleaves at the carboxyl side of Lysine residue when it is preceded by four Aspartic acids (Asp-Asp-Asp-Asp-Lys) and is not followed by Proline. Enterokinase can be used to remove the fusion tag of a protein or the N-terminal pro-sequence of a precursor protein that is genetically engineered to be activable with enterokinase.
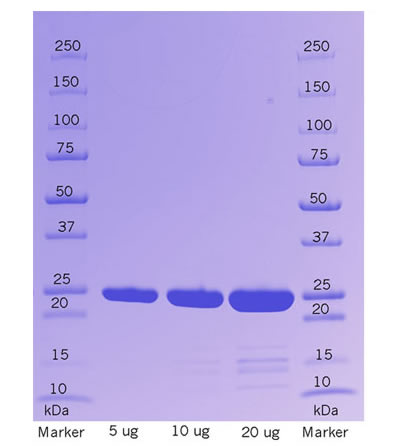
Recombinant Bovine Enterokinase is produced from CHO. and contains no trace of contaminating proteases. The enzyme is purified by a proprietary process to obtain high purity and maximum enzymatic activity. The product is delivered as clear aqueous solution (≥ 5 U/μL) in a storage buffer specifically formulated to ensure the highest enzyme stability during storage.
The enzyme is highly active at 4°C, allowing digestion to be performed at a desirable low temperature for maximum stability of temperature-sensitive target substrates during digestion.
Unit definition: One Unit is defined as the amount of enzyme required to cleave 0.5 mg of a DDDDK-containing protein (~30 kDa in size) to 95% completion in 12 to 16 hours at 25°C.

.jpg)