RFP (Red fluorescent protein) cloned from Discosoma coral (DsRed or drFP583) is an important tool in biotechnology and cell biology as a spectrally distinct companion or substitute for the green fluorescent protein (GFP; from Aequorea jellyfish).
RFP-Catch-Aga™ Anti-RFP Alpaca nanobody conjugated Agarose Beads
Catalog number :AT1769
- Overview
- Description
- RFP-Catch-Aga™ Anti-RFP tag nanobody (from alpaca, VHH, Single Domain antibody) conjugated Agarose Beads
- Reactivity
- exogenously overexpressed RFP fusion proteins in cells or E. coli.Species independent
- Tested applications
- Immunoprecipitation (IP),Co-Immunoprecipitation (Co-IP),Chromatin Immunoprecipitation (ChIP)RNA Binding Protein Immunoprecipitation (RIP)Enzyme assaysMass spectrometryAffinity purificationIn IP, 25 µL of beads suspension for ~500 µL of crude total cell lysate solution.Optimal dilutions/concentrations should be determined by the end user.
- Specificity
- This antibody detects RFP-tagged proteins exogenously expressed in cells. Please note that the RFP tags add approximately 28 kDa to the molecular weight of the fusion protein.
- Properties
- Immunogen
- Full-length RFP protein
- Clonality
- Monoclonal, nanobody
- Isotype
- Alpaca single domain antibody (nanobody, VHH)
- Form
- Agarose beads Conjugated anti- RFP Nanobody, the product is supplied as a 50% suspension in 10 mM phosphate buffered saline (PBS), pH 7.4, and 0.02% (w/v) sodium azide as preservative.
- Storage instruction
- Store at +4°C and do not freeze.
- Host
- Alpaca

- Structure Image
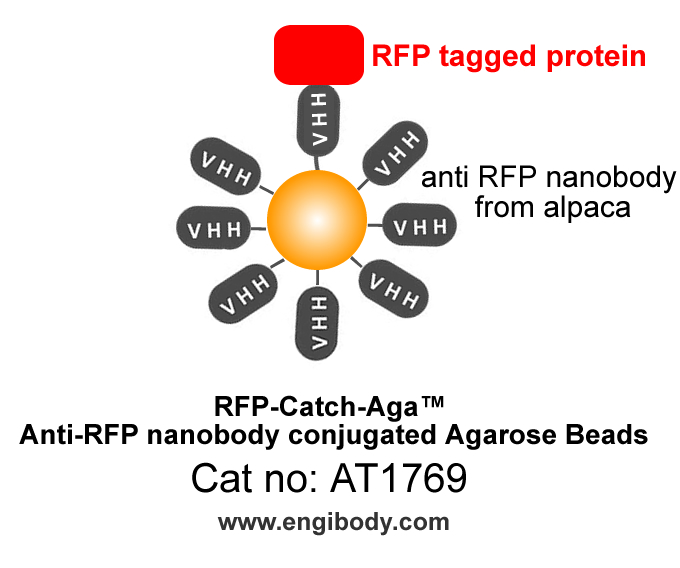
- Beads Diameter
- ~ 90 µm (cross-linked 8% agarose beads)
- Binding Capacity
- 25 µg of RFP can be precipitated with 25 µL of beads suspension.
- Positive Controls
- exogenously expressed RFP proteins in cells or E. coli.
- Applications
- Application Image
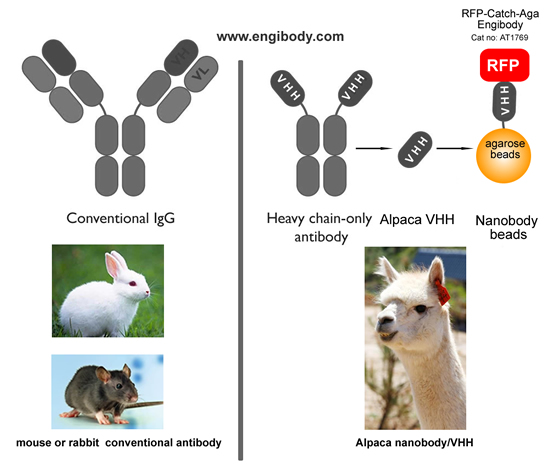
Figure 1. A schematic representation of benefits (Alpaca Antibody Advantage)- No interfering of traditional antibody heavy and light chains in your downstream WB and mass spectrometry analysis
- One step immunoprecipitation
- Highly specific binding
- Low background
- High affinity
- High stability
- Easy elution of native proteins
- Highlights
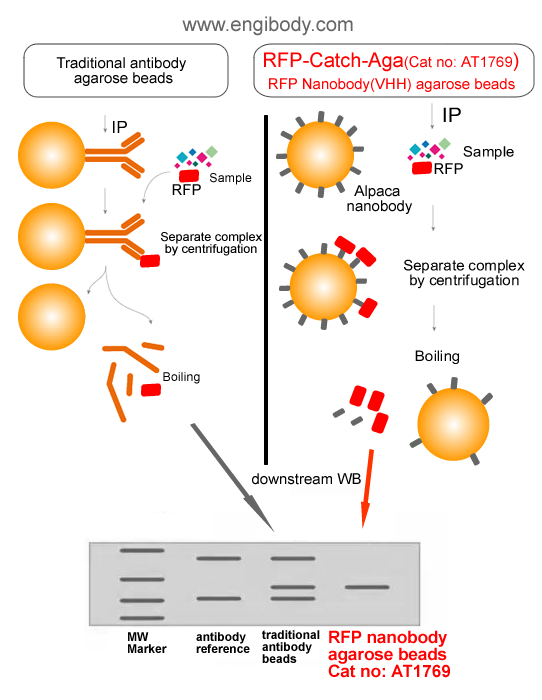
Figure 2. A schematic representation of benefits (no interfering of traditional antibody heavy and light chains)
- Protocols
Protocol for Immunoprecipitation (IP/CoIP) of RFP-Fusion Proteins from mammalian cell lysate
Solutions and Reagents1X Cell Lysis Buffer: 50 mM Tris (pH 7.5), 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1% Triton X-100. Recommend adding 1 mM PMSF before use.1X Wash Buffer: TBS (50 mM Tris HCl, 150 mM NaCl, pH 7.5)5X SDS Sample Loading BufferProcedure1. Harvest cells1). For one immunoprecipitation reaction the use of ~106 - 107 mammalian cells (approx. one 10-cm dish) expressing a RFP-tagged protein of interest is recommended. To harvest adherent cells, aspirate growth medium, add 1 mL ice-cold PBS to cells and scrape cells from dish. Transfer cells to a pre-cooled tube, spin at 500 g for 3 min at +4°C and discard supernatant. Wash cell pellet twice with ice-cold PBS, gently resuspending the cells.2. Lyse cells1). Resuspend cell pellet in 500 µL ice-cold lysis buffer by pipetting.Note: Add protease inhibitors and 1 mM PMSF into lysis buffer.Attention: For nuclear/chromatin proteins: Use RIPA buffer added with 1 mg/mL DNase, 2.5 mM MgCl2, protease inhibitors and 1 mM PMSF.2). Place the tube on ice for 30 min with extensively pipetting every 10 min.3). Centrifuge cell lysate at 14000x g for 10 min at +4°C. Transfer lysate(supernatant) to a pre-cooled tube. Discard pellet.Note: At this point cell lysate may be put at -80°C for long-term storage.3. Equilibrate beads1). Resuspend the beads by inverting the product tube or gently pipetting up and down. Do not vortex the agarose beads,2). Pipette 25 µL bead slurry into 500 µL ice-cold cell extract. Centrifuge at 2500x g for 2 min at +4°C. Discard supernatant and repeat wash twice.4. Bind proteins1). Add 25 µL equilibrated anti-RFP agarose beads slurry (step 3) into 500 µL cell extract (step 2). Save 30 µL of cell extract for input of immunoblot analysis before IP. Incubation at a rotator for 1-3 hours at 4°C or overnight 4°C.2). Centrifuge at 2500x g for 2 min at +4°C. Discard supernatant.5. Wash beads1). Resuspend protein-antibody-beads complex in 1mL ice-cold wash buffer. Centrifuge at 2500x g for 5 min at +4°C. Discard supernatant and repeat wash at least three times.6. Elute proteinsTwo elution methods are recommended according to protein characteristics or further usage:Option A: Native ElutionElution under acidic conditions with 0.2 M glycine (pH 2.5). This is a fast and efficient elution method. Neutralization of the eluted proteins with neutralizing buffer (1 M Tris, pH 10.4) may help preserve its activity. Neutralizing buffer needs to be placed in the collection tube in advance.Attention: It is necessary to make a preliminary experiment in advance to determine how much neutralization buffer is needed to neutralize glycine (PH 2.5)Option B: Denaturing Elution for SDS-PAGEElution with sample loading buffer under denaturing conditions for gel electrophoresis and immunoblotting.A: Elution with 0.2 M Glycine (pH 2.5) - The procedure should be performed at room temperature.
Note:Do not leave the beads in this buffer more than 20 minutes.1. Add 50 µL of 0.2 M Glycine (pH 2.5), to each sample and control beads complexes.2. Incubate the samples and controls with constantly pipette up and down for 1-3 minutes at room temperature. (Note: Do not turn the tube upside down to prevent the complex from sticking to the tube wall)3. Centrifugate the beads complex at 2500x g for 5 min at 4°C. Transfer the supernatants to fresh tubes containing about 5 µL of neutralizing buffer. Be careful not to transfer any beads.4. Repeat steps 1 – 3 in order to improve elution efficiency, pooling eluates in same tube or collect eluate through different tubes.6. For immediate use, store the eluates at 4 °C. Store at –20 °C for long term storage.B: Elution with SDS-PAGE Sample Loading Buffer1. Resuspend each sample with 30 µL 1X SDS sample loading buffer (6 µL 5X SDS sample loading buffer can be added into 24 µL cell lysis buffer). Vortex.2. Boil the sample and control tubes for 10 minutes at 95 – 100°C to dissociate immunocomplex from anti-RFP agarose beads.3. Anti-RFP agarose beads immunocomplex can be centrifugated at 2500x g for 2 min at 4°C. Transfer the supernatants to fresh tubes. The samples and controls are ready for loading on SDS-PAGE and immunoblotting using anti-RFP or specific antibodies against the fusion protein.
Related Products
| Catalog number | AT1765 |
| Catalog number | AT1795 |
| Catalog number | AT1773 |
Reviews
loading...